Describe the Structure and Properties of Water
A combination of two or more atoms bound together that has different qualities than the individual atoms. Water is a simple molecule composed of two small positively charged hydrogen atoms and one large negatively charged oxygen atom.

Water Molecules Molecular Structure Physical Properties Earth Surface
When the hydrogens bind to the oxygen it creates an asymmetrical molecule with positive charge on one side.

. Describe the structure of water its properties and its importance to life. Water H2O is also a compound. H 2 O that contains two hydrogen atoms each sharing a pair of electrons with an oxygen atom see Figure 1.
H side of the molecule is positive. Molecular geometry of water is bent in structure resulting from Td electron pair geometry. Water show electron pairing structureit has 4 regi View the full answer.
Water has high specific heat and high heat of Vaporization. The most important chemical and physical features of water are summariedin this Fig. This is due to cohesion of water molecules.
The molecules that attract water molecules the most are those with a full charge as an ion. Water is a polar molecule which means it can attract other polar molecules. Some choices may be used more than once.
Include in your answer. As we know water has. Both of these properties are due to requirement of.
The geometry of the water molecule is dictated by the shapes of the outer electron orbitals of the oxygen atom which are similar to the bonding orbitals of carbon. Water H 2O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid which is nearly colorless apart from an inherent hint of blue. Water molecules are polar so they form hydrogen bonds resulting in unique properties.
This is not inconsiderable and energy is required to break the bonds or is yielded by the formation of hydrogen bonds. 23 rows Properties of water include its chemical formula H2O density melting boiling point how one. The reason for the polarity of water due to the electronegativity of hydrogen and oxygen waters liquid nature and how molecules become dissolved in water b.
The Properties of Water. Water is the most abundant compound on Earths surface. Studies have shown that clustering of water molecules occurs in solutions because of so-called hydrogen bonds weak interaction which are about 10 of the covalent water bond strength.
The importance of partner exchange to pH and heat capacity c. Water can moderate temperature because of the two properties. O is very electronegative hogs electrons is negative.
This indicates how strong in your memory this concept is. Some are essential for life while others have profound effects on the size and. In nature water exists in the liquid solid and gaseous states.
Water properties Complete the following statements to describe the structure and properties of water. At room temperature approximately 25 degrees Celsius it is a tasteless odorless and colorless liquid. High-specific heat and the high heat of vaporization.
Chemical Formula of water as we are all aware is H2O. These orbitals describe a rough tetrahedron with a hydrogen atom at each of the two corners and un. However the electrons of the covalent bonds are not shared equally between the oxygen and hydrogen.
Water has high tension. Many of waters roles in supporting life are due to its molecular structure and a few special properties. It is the most abundant substance on Earth and the only common substance to.
Structure of Water molecule. Surface tension heat of vaporization and vapor pressure. The human body is comprised of over 70 waterand it is a major component of many body fluids including bloodurineand saliva.
Physical Properties of Water. Water is a molecule. Thermal properties of water.
Water is a major component of all living things. In a water molecule each hydrogen atom shares an electron pair with the oxygen atom. Describe the structure and geometry of a water molecule and explain what properties emerge as a result of this structure.
Boiling and freezing points. Adhesion in water oxygen shares a bond with two hydrogen atoms Oxygen is more than hydrogen making the bond with the oxygen being the negative end and the two hydrogens being the positive end. Due to this property small organism float or walk on water surface.
Dscription of structure and geometry of water molecule Water molecule consist of 2 H atoms and one O atom. Two need filling two O-H bonds form at two corners of the tetrahedron. It is anomalous in many of its physical and chemical properties.
When atoms share electrons in. Structure and properties of water 1. The molecule of water has covalent bonding between Hydrogen and Oxygen atoms.
The key to understanding properties of water are the hydrogen bonds. Water molecules form a lot of hydrogen bonds between one. Water is essential for lifeIt covers 23 of the earths surface and every living thing is depend upon it.
Water freezes and boils at lower temperatures than other similar compoundsFig water is a polar molecule Fig concept of hydrogen bonds Fig heat capacity. It is in dynamic equilibrium between the liquid and gas states at 0 degrees Celsius and 1 atm of pressure. It is by far the most studied chemical compound and is described as the universal solvent and the solvent of life.
Water is colorless odorless and tasteless liquid in its natural state. It can form hydrogen bonds with other elements. The reasons that temperature and ion concentration affect.
This makes water an extremely potent solvent. Practice Structure and Properties of Water. The Structure of Water.
Outer shell of O has four orbitals as a tetrahedron. High-specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius. Its structure consists of two hydrogen atoms joined to one oxygen atom by single covalent bonds.
The level of polarity in water is extremely high uniquely so.

Chemical Principles Properties Of Water Biochemistry Biology Notes Biochemistry Study Tips College

Pin On Nosnegroj Nai Wiccan Dragon Satanic Hierarchy
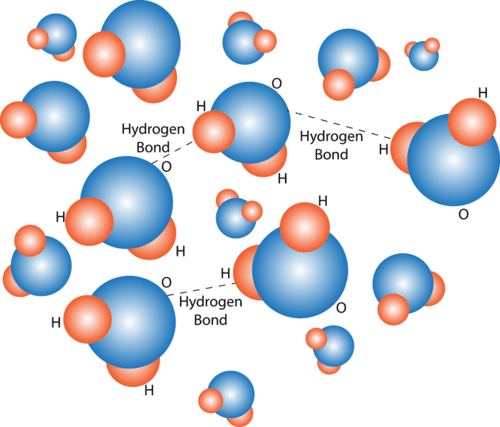
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation

007 Hydrogen Bonding Water Hydrogen Bond Bond Molecules

Properties Of Water Task Cards Task Cards Formative Assessment Task

The Structure And Properties Of Water Introduction To Chemistry

Protein Tertiary Structure Wikipedia Learn Biology Structural Biology Chemical Kinetics

Properties Of Water Anchor Chart Matter Science Science Anchor Charts Matter Anchor Chart

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Teaching Chemistry

Ks4 Aqa Gcse Chemistry Science Natural Polymers Lesson Activities Teaching Resources Gcse Chemistry Natural Polymers Chemistry Lessons

Properties Of Water Task Cards Task Cards High School Biology Teacher

Lesson Summary Water And Life Article Khan Academy

What Are The 5 Postulates Of Dalton S Atomic Theory Atomic Theory Chemistry Lessons Study Chemistry

The Structure And Properties Of Water Introduction To Chemistry

The Structure And Properties Of Water Introduction To Chemistry

Properties Of Water Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Water Lessons Science Lessons Chemistry Lesson Plans

Properties Of Water Biology Doodle Diagram High School Biology Lessons Biology Doodles


Comments
Post a Comment